Cdcl3 Peak In H Nmr
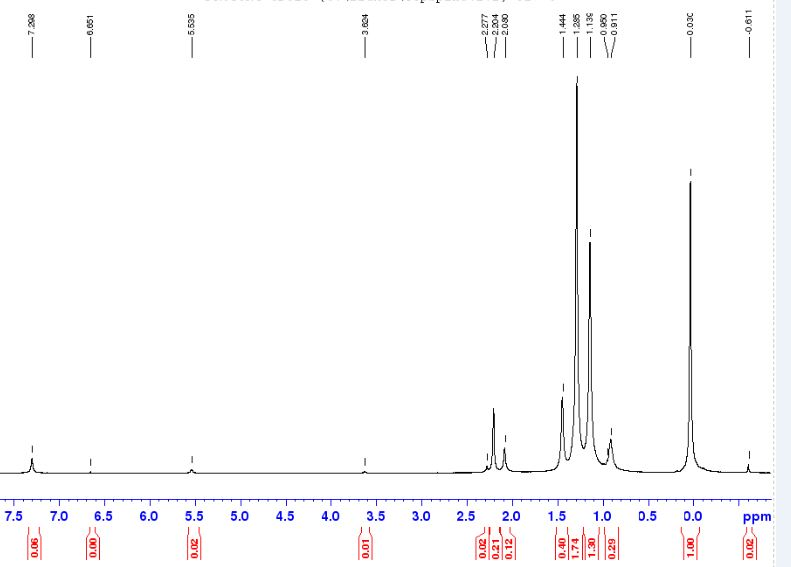
Cdcl3 Peak In H Nmr. Reference or download our nmr shifts charts for the most common deuterated solvents. Similarly introduction of further higher number. There are three isotopes of hydrogen used in nmr spectroscopy: In spectra recorded with deuterated chloroform (cdcl3) as the lock solvent, the three peaks at = 75 are due to splitting of the. The nmr spectra on this page have been produced from data taken from the spectral data base system for organic compounds (sdbs) at the national institute of materials and chemical research in japan. 300 mhz, 400 mhz and if two protons are magnetically inequivalent, there are two peaks in the spectrum for each proton. Unique or peculiar spin coupling patterns, making these especially contain one h atom. But in 13c nmr of dmso its peak appeared as septate.so why the intensity varies. But in 13c nmr of dmso its peak appeared.
The thing is that i read somewhere that the peak should be at 7.24 ppm but there is a lot of peak in this region of my spectra so how can i determine where is it exactly. H nmr carbon nmr ranges diethyl ether nmr. Reference or download our nmr shifts charts for the most common deuterated solvents. Proton nmr spectroscopy peak analysis using c3h7cl. Since the 1d carbon experiment is highly susceptible to the 13c nuclei in the.

Nmr water signals signals for water occur at different frequencies in 1 h nmr spectra depending on the solvent used.
The nmr spectra on this page have been produced from data taken from the spectral data base system for organic compounds (sdbs) at the national institute of materials and chemical research in japan. Peaks in the 13c nmr spectra corresponding to the deuterated solvent molecules show. Consider the chemical shifts, integrals(displayed on the top of each signal) and multiplicities. Nmr water signals signals for water occur at different frequencies in 1 h nmr spectra depending on the solvent used. Part of proton spectrum of strychnine in cdcl3. Next to each h on the structure above, indicate the peak multiplicity expected by coupling (using s = singlet, d = doublet, t = triplet, q = quartet; Shows the magnetic field strength of the nmr is roughly 7.046 t. The solvent used was cdcl3, so a peak from h2o might be expected around 1.6 ppm, but this wouldn't correspond to either of the aforementioned peaks. 19f is an important nucleus for nmr spectroscopy because of its receptivity and large chemical shift dispersion. Top suggestions for cdcl3 nmr peak. When we do an nmr experiment with these solvents the solvent peaks stick out like dogs' balls in the carbon spectrum. There are three isotopes of hydrogen used in nmr spectroscopy: Proton nmr spectroscopy peak analysis using c3h7cl.
H nmr carbon nmr ranges diethyl ether nmr. Top suggestions for cdcl3 nmr peak. The nmr spectra on this page have been produced from data taken from the spectral data base system for organic compounds (sdbs) at the national institute of materials and chemical research in japan. Nmr peaks have a shape that is called lorentzian. Peaks in the 13c nmr spectra corresponding to the deuterated solvent molecules show. When we do an nmr experiment with these solvents the solvent peaks stick out like dogs' balls in the carbon spectrum. $cdcl_{3}$ is therefore commonly used as a solvent.

Consider the chemical shifts, integrals(displayed on the top of each signal) and multiplicities.
Whenever you run a #^13c# spectrum in cdcl₃, you always get a triplet solvent peak at 77.5 ppm. Since the 1d carbon experiment is highly susceptible to the 13c nuclei in the. Proton spectrum of 0.1% ethylbenzene in cdcl3 taken on a varian unity 400 mhz spectrometer. Part of proton spectrum of strychnine in cdcl3. Select one exersise from the left table(click) 2. Nuclear magnetic resonance study of exchanging systems. When we do an nmr experiment with these solvents the solvent peaks stick out like dogs' balls in the carbon spectrum. There are three isotopes of hydrogen used in nmr spectroscopy: Figure 7 shows the 13c nmr spectra of 1 m lidocaine in cdcl3. The amount of energy, and hence the exact frequency of em radiation required for resonance to occur is dependent on both the strength of the magnetic field applied and. But in 13c nmr of dmso its peak appeared. Unique or peculiar spin coupling patterns, making these especially contain one h atom. Nuclear magnetic resonance (nmr) in chemistry investigates the electron density from the behaviour of the nucleus under static and an variable magnetic field of molecules. Peaks in the 13c nmr spectra corresponding to the deuterated solvent molecules show.
Sh ow t h eir degr ee of va r ia bilit y. 300 mhz, 400 mhz and if two protons are magnetically inequivalent, there are two peaks in the spectrum for each proton. Occa sion a lly, in or der t o dist in gu ish bet ween pea ks wh ose a ssign m en t wa s. 19f is an important nucleus for nmr spectroscopy because of its receptivity and large chemical shift dispersion. Other differences may be caused by variations in the nuclear overhauser effect. (2) does the spectrum have 9 signals in it? I'm analyzing the h nmr spectrum for an unknown compound that was formed from the esterification reaction of acetic acid with an unknown alcohol. Select one exersise from the left table(click) 2.
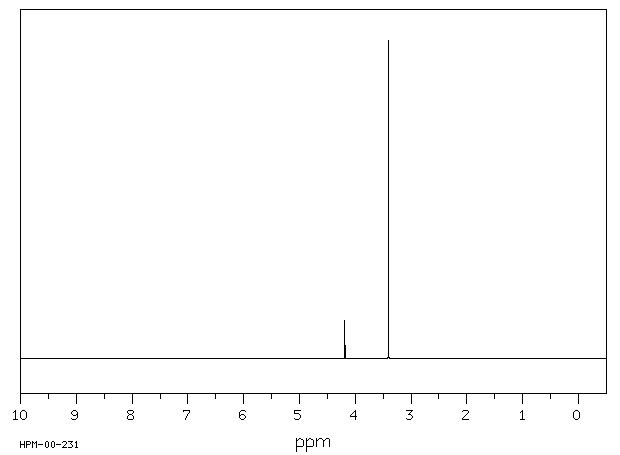
Analyse the molecular formula of the target molecule (displayed over the.
Reference or download our nmr shifts charts for the most common deuterated solvents. These tables can support you in identifying and separating nmr signals of impurities that might originate from residual solvents or from your reaction 1h nmr chemical impurity shifts table. Other differences may be caused by variations in the nuclear overhauser effect. In spectra recorded with deuterated chloroform (cdcl3) as the lock solvent, the three peaks at = 75 are due to splitting of the. 300 mhz, 400 mhz and if two protons are magnetically inequivalent, there are two peaks in the spectrum for each proton. Similarly introduction of further higher number. But in 13c nmr of dmso its peak appeared. Proton nmr spectroscopy peak analysis using c3h7cl. Figure 7 shows the 13c nmr spectra of 1 m lidocaine in cdcl3. Most nmr spectra are recorded for compounds dissolved in a solvent. Unique or peculiar spin coupling patterns, making these especially contain one h atom. Part of proton spectrum of strychnine in cdcl3. The peak position in hz.
To avoid spectra dominated by the solvent signal, most 1h nmr spectra are recorded in a in chloroform solvent (cdcl3), this corresponds to chcl3, so a singlet signal is observed at 726 cdcl3 peak. To begin with, the nmr spectrometer must be tuned to a specific nucleus, in this case the proton.
 Source: www.researchgate.net
Source: www.researchgate.net These tables can support you in identifying and separating nmr signals of impurities that might originate from residual solvents or from your reaction 1h nmr chemical impurity shifts table.
 Source: www.researchgate.net
Source: www.researchgate.net To begin with, the nmr spectrometer must be tuned to a specific nucleus, in this case the proton.
 Source: www.researchgate.net
Source: www.researchgate.net Peaks in the 13c nmr spectra corresponding to the deuterated solvent molecules show.
 Source: d2vlcm61l7u1fs.cloudfront.net
Source: d2vlcm61l7u1fs.cloudfront.net To begin with, the nmr spectrometer must be tuned to a specific nucleus, in this case the proton.
The amount of energy, and hence the exact frequency of em radiation required for resonance to occur is dependent on both the strength of the magnetic field applied and.
 Source: www.researchgate.net
Source: www.researchgate.net Analyse the molecular formula of the target molecule (displayed over the.
.jpg) Source: www.azom.com
Source: www.azom.com The thing is that i read somewhere that the peak should be at 7.24 ppm but there is a lot of peak in this region of my spectra so how can i determine where is it exactly.
 Source: i1.rgstatic.net
Source: i1.rgstatic.net There are three isotopes of hydrogen used in nmr spectroscopy:
 Source: d2vlcm61l7u1fs.cloudfront.net
Source: d2vlcm61l7u1fs.cloudfront.net Select one exersise from the left table(click) 2.
 Source: www.researchgate.net
Source: www.researchgate.net The nmr spectra on this page have been produced from data taken from the spectral data base system for organic compounds (sdbs) at the national institute of materials and chemical research in japan.
These tables can support you in identifying and separating nmr signals of impurities that might originate from residual solvents or from your reaction 1h nmr chemical impurity shifts table.
 Source: images.squarespace-cdn.com
Source: images.squarespace-cdn.com $cdcl_{3}$ is therefore commonly used as a solvent.
 Source: www.researchgate.net
Source: www.researchgate.net When we do an nmr experiment with these solvents the solvent peaks stick out like dogs' balls in the carbon spectrum.
 Source: www.quizover.com
Source: www.quizover.com What is this peak due to and why the heck is it there?
 Source: www.researchgate.net
Source: www.researchgate.net Part of proton spectrum of strychnine in cdcl3.
 Source: d2vlcm61l7u1fs.cloudfront.net
Source: d2vlcm61l7u1fs.cloudfront.net Consider the chemical shifts, integrals(displayed on the top of each signal) and multiplicities.
 Source: i.stack.imgur.com
Source: i.stack.imgur.com Select one exersise from the left table(click) 2.
 Source: s3-us-west-2.amazonaws.com
Source: s3-us-west-2.amazonaws.com I'm analyzing the h nmr spectrum for an unknown compound that was formed from the esterification reaction of acetic acid with an unknown alcohol.
 Source: www.researchgate.net
Source: www.researchgate.net The peak position in hz.
 Source: www.umich.edu
Source: www.umich.edu Select one exersise from the left table(click) 2.
 Source: www.researchgate.net
Source: www.researchgate.net Select one exersise from the left table(click) 2.
 Source: www.researchgate.net
Source: www.researchgate.net Whenever you run a #^13c# spectrum in cdcl₃, you always get a triplet solvent peak at 77.5 ppm.
 Source: d2vlcm61l7u1fs.cloudfront.net
Source: d2vlcm61l7u1fs.cloudfront.net Similarly introduction of further higher number.
 Source: www.researchgate.net
Source: www.researchgate.net Nmr peaks have a shape that is called lorentzian.
 Source: www.researchgate.net
Source: www.researchgate.net Figure 7 shows the 13c nmr spectra of 1 m lidocaine in cdcl3.
 Source: www.researchgate.net
Source: www.researchgate.net However, whenever cdcl3 is used as an nmr solvent, a small singlet is always observed at 7.26 delta.
 Source: www.researchgate.net
Source: www.researchgate.net 19f is an important nucleus for nmr spectroscopy because of its receptivity and large chemical shift dispersion.
 Source: www.researchgate.net
Source: www.researchgate.net The amount of energy, and hence the exact frequency of em radiation required for resonance to occur is dependent on both the strength of the magnetic field applied and.
 Source: www.researchgate.net
Source: www.researchgate.net Next to each h on the structure above, indicate the peak multiplicity expected by coupling (using s = singlet, d = doublet, t = triplet, q = quartet;
.jpg) Source: www.azom.com
Source: www.azom.com Next to each h on the structure above, indicate the peak multiplicity expected by coupling (using s = singlet, d = doublet, t = triplet, q = quartet;
 Source: www.researchgate.net
Source: www.researchgate.net 19f is an important nucleus for nmr spectroscopy because of its receptivity and large chemical shift dispersion.
 Source: i.stack.imgur.com
Source: i.stack.imgur.com 19f is an important nucleus for nmr spectroscopy because of its receptivity and large chemical shift dispersion.
 Source: chem.ch.huji.ac.il
Source: chem.ch.huji.ac.il Since the 1d carbon experiment is highly susceptible to the 13c nuclei in the.
 Source: www.researchgate.net
Source: www.researchgate.net Proton spectrum of 0.1% ethylbenzene in cdcl3 taken on a varian unity 400 mhz spectrometer.
 Source: www.researchgate.net
Source: www.researchgate.net Figure 7 shows the 13c nmr spectra of 1 m lidocaine in cdcl3.
Posting Komentar untuk "Cdcl3 Peak In H Nmr"